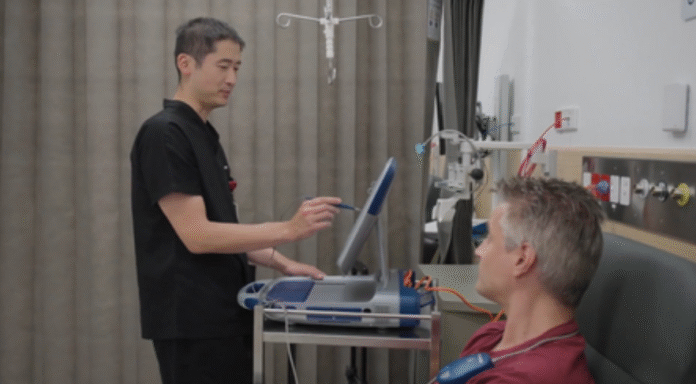
MELBOURNE — Scientists in Australia have led the world’s first human trial of a one-time gene-editing therapy designed to sharply reduce levels of LDL (bad) cholesterol and triglycerides in patients with hard-to-treat lipid disorders.
The treatment, known as CTX310, uses CRISPR-Cas9 gene editing delivered through fat-based nanoparticles that target the liver and switch off the ANGPTL3 gene. Disabling this gene can lower both LDL cholesterol and triglycerides, two major contributors to heart disease, Monash University said on Monday.
The Victorian Heart Hospital, operated by Monash Health in partnership with Monash University, treated three of the 15 participants enrolled in phase one of the trial. The study involved patients aged 18 to 75 across Australia, New Zealand and the United Kingdom.
At the highest tested dose, a single administration of CTX310 reduced LDL cholesterol by an average of 50 percent and triglycerides by 55 percent. These reductions were sustained for at least 60 days after treatment. Across multiple dose levels, participants saw reductions of nearly 60 percent, with only mild and temporary side effects reported.
According to findings published in the New England Journal of Medicine, CTX310 is the first therapy to achieve large and simultaneous reductions in both LDL cholesterol and triglycerides, positioning it as a potential option for people with mixed lipid disorders.
“The possibility of a single-course treatment with lasting effects could be a major step in how we prevent heart disease,” said Stephen Nicholls, director of the Victorian Heart Hospital and the trial’s lead investigator. “It makes treatment easier, reduces ongoing costs, and relieves pressure on the health system, all while improving a person’s quality of life.”
Researchers plan to conduct larger trials in more diverse patient groups to further evaluate the therapy’s long-term safety and effectiveness. (Source: IANS)