NEW DELHI– An improved version of an experimental immunotherapy drug has shown encouraging results against aggressive cancers in a Phase 1 clinical trial, according to researchers.
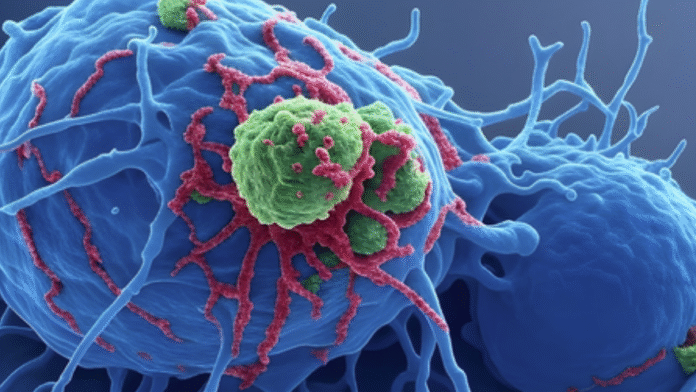
CD40 agonist antibodies — a class of cancer drugs — have long been effective at activating the immune system to destroy tumors in animal models. But in humans, even at low doses, they have triggered serious side effects, including dangerous systemic inflammation, low platelet counts, and liver toxicity.
In 2018, a team of U.S. scientists led by Rockefeller University modified a CD40 agonist antibody to boost its potency while reducing its toxicity. The resulting drug, called 2141-V11, was tested in a small trial involving 12 patients. Six saw their tumors shrink, including two whose tumors disappeared entirely.
“Seeing these significant shrinkages and even complete remission in such a small subset of patients is quite remarkable,” said first author Juan Osorio, a medical oncologist at Memorial Sloan Kettering Cancer Center.
The effects weren’t limited to tumors injected with the drug — tumors elsewhere in the body also shrank or were destroyed by immune cells, the researchers reported in Cancer Cell. “This effect — where you inject locally but see a systemic response — that’s not something seen very often in any clinical treatment,” said study leader Jeffrey V. Ravetch of Rockefeller University. “It’s another very dramatic and unexpected result from our trial.”
CD40 is a receptor found on immune cells and is part of the tumor necrosis factor (TNF) receptor superfamily. When activated, it stimulates the immune system to mount an anti-tumor response and produce tumor-specific T cells.
Ravetch’s lab engineered 2141-V11 to bind more tightly to human CD40 receptors and enhance crosslinking by engaging a specific Fc receptor, making it 10 times more effective in triggering an anti-tumor immune response. Instead of being given intravenously, the drug was injected directly into tumors.
The trial included patients with melanoma, renal cell carcinoma, and various types of breast cancer. None experienced the severe side effects typically associated with earlier CD40 drugs. Six patients had systemic tumor reductions, and two — one with melanoma and one with breast cancer — achieved complete remission. (Source: IANS)